Towards evaluation of meat digestibility
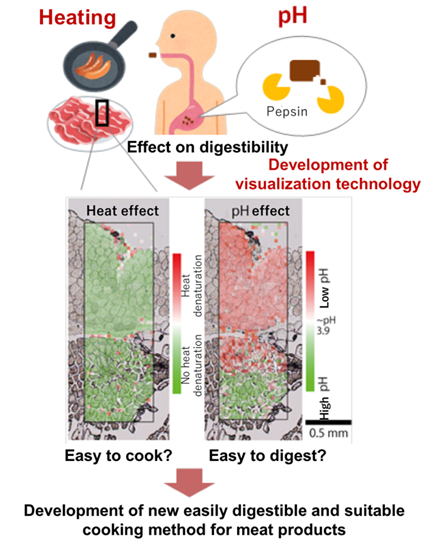
An infrared micro spectroscopic approach that could simultaneously detect and visualize the effects heat and pH on proteins involved in the digestion of meat has been developed. Analysis of the target sites in meat tissues facilitates the measurement of pH change due to gastric acid in an environment that simulates the stomach and the thermal change of food proteins in the cooking process. This technology is useful for developing an appropriate cooking method that does not reduce the digestibility of meat as well as for developing easily digestible meat products.
Overview
Malnutrition among elderly people can be improved effectively by adequate consumption of meat which is a good source of protein. It is known that the digestibility of the gastric digestive enzyme pepsin decreases when the meat protein is overheated during the cooking process or when the pH does not decrease sufficiently in the stomach. In order to clarify the appropriate cooking methods of meat products and to contribute to the development of products with excellent digestion and heat absorption properties, the Institute of Livestock and Grassland Science, NARO (NILGS), the Research Unit "Quality of Animal Products" (UR 370) from the French National Institute for Agricultural Research (INRA), and the "Spectroscopy and Microscopy in the Infrared using Synchrotron" (SMIS) beamline of SOLEIL Synchrotron (France) have jointly developed an infrared microspectroscopic approach that could easily and simultaneously detect the effects of heat and pH of proteins in meat samples before and after cooking. The technology also allows to target the analyzing site in meat tissues and is therefore useful for visualization of pH change due to gastric acid in an environment that simulates the stomach as well as and the thermal behavior of food protein in the process of cooking.
Publication
Motoyama M1, Venien A2, Loison O2, Sandt C3, Watanabe G1, Sicard J2, Sasaki K1, Astruc T1. In situ characterization of acidic and thermal protein denaturation by infrared microspectroscopy. Food Chemistry 248:322-329. DOI: https://doi.org/10.1016/j.foodchem.2017.11.031
1 Institute of Livestock and Grassland Science, NARO (NILGS)
2 UR 370, National Institute of Agricultural Research (INRA)
3 SMIS Beamline, SOLEIL Synchrotron




